In his 1919 paper A New Apparatus for Measuring Surface Tension, J. Gen Physiol., 1, 521-524, Lecomte du Nouy pointed out the large discrepancies in results obtained with methods that had been applied prior to that time in determining the surface tensions of pure liquids and presented his new technique. Some of the other techniques were oscillating jet (dynamic), hanging drop, spinning drop, sessile drop, capillary rise and maximum bubble pressure. The key to du Nouy's new apparatus was the use of a torsion wire, which could be twisted to apply torque to a lever arm, from which a ring of interest was suspended in contact with the liquid. In the original design, du Nouy proposed simply calibrating the instrument by measuring the force maximum pull of pure liquids for which surface tension was known, making the technique useful but still relative. It was not until 1930 that first Harkins and Jordan and then Freud and Freud published works very carefully analyzing the theory associated with the du Nouy ring. They determined that the technique could be used as a very accurate absolute method. The total force acting on the ring, Wtot, can be calculated as
Wtot = Wring + 4pRgideal, Equation 1
Where Wring is the weight of the ring, R is the radius of the ring, and gideal the surface tension.
Harkins and Jordan determined that an empirical correction factor was required because the size (and shape) of the surface inside and outside the ring was not the same, therefore the vertical force components acting on the ring were not symmetrical. The correction factor, f, was determined as a function of the size of the ring, radius R, and the size of the wire of which the ring was made, radius r.
g = gideal x f(R3/V, R/r), Equation 2
In 1941, it was pointed out by Zuidema and Waters Ring Method for the Determination of Interfacial Tension, Indus. Eng. Chem., 13(5), 312-313, that an additional correction factor F was required in cases when the difference between the densities of the two phases making the interface was small or when the scale readings were relatively high.
The interfacial tension of the sample is calculated accordingly by means of the following equation:
Interfacial tension (dyn/cm) = P x F , Equation 3
Where P is the scale reading when the film ruptures (in dynes per centimeter), and F is the factor converting the scale reading (in dynes per centimeter) to interfacial tension, as obtained from Equation 4. R/r is the value of the diameter ratio for the ring.
The value of F is obtained as follows:
F = 0.7250 + [1.452P/C�(D - d) + 0.04534 - 1.679/(R/r)]� , Equation 4
Where C is the circumference of the ring in mm; D is the density of the aqueous layer at 25�C, in grams per milliliter; d is the density of the organic layer for interfacial testing at 25�C, in grams per milliliter; R is the radius of ring, in millimeters; and r is the radius of the wire of the ring, in millimeters.
Zuidema and Waters were mainly investigating the interfacial tension between hydrocarbon and water phases. In these cases, the density difference, Dr, was much lower (rwater = 1.00 g cm-3, rdecane = 0.73 g cm-3, rdecane = 0.75 g cm-3, rhexadecane = 0.77 g cm-3) than in surface tension measurements where one phase is air (rair = 0.001 g cm-3).
Wilhelmy used the same hardware but a plate instead of a ring to measure the surface or interfacial tension as a function of the weight of the liquid drawn by a plate when the plate is lifted from or through the surface of liquid. Wilhelmy did not attempt to discuss a detailed calculation of surface tension. The Wilhelmy plate method does not require a correction factor. The force acting on the plate is simply the sum of the surface effect and the buoyant effect of a column of water pulled up under the plate (if the contact angle of the liquid with the plate is zero);
Wtot = Wplate + pgideal , Equation 5
Where p is the horizontal perimeter (2 x length + 2 x thickness) of the plate. A buoyant force, DrghA (where h is the height above or below the free liquid level and A is the cross-sectional area of the plate), is added or subtracted if the plate is above or below the level of the free liquid. The subsequent method we use now employs a thin vertical plate, usually a glass slide or a platinum blade of known perimeter, attached to a microbalance. Static measurements can be performed by lowering a dry unwetted glass plate to the liquid surface until its lower edge nearly touches the surface. At this point, the liquid jumps onto the edge and sides of the plate. The liquid wets the plate perimeter and increases its total mass to some maximum point which is proportionnal to the surface tension or interfacial tension of liquid (Assuming a zero contact angle, the weight of the meniscus formed on the perimeter of the plate is divided by the perimeter of the plate's perimeter). Dynamic measurements may be performed by immersing completely a platinum plate with defined geometrical dimensions and micro-roughened surface (for optimal wetting properties) into the sample liquid. The plate is then pulled out through the surface of the liquid until maximum force is reached when the plate brakes away from the surface. A similar approach is used to measure the contact angle formed when the plate is immersed in a liquid of known surface tension.
The TSD 971 performs according to the ASTM D971 Standard Test Method a static measurement under rigidly standardized non-equilibrium conditions of the torque necessary to detach a planar du Nouy ring upward from the surface of water, as well as upward from the water-oil interface. The resulting surface and interfacial tensions are calculated in mN/m or Dyne/cm by means of a calculator or a supplied Excel datasheet out of the measured torque values in mg/mm, the densities of water and oil in g/ml, and the geometrical parameters of the ring. In addition and for optional control tests, the TSD 971 may be supplied with both a ring and optional plates, in order to measure alternatively and to display directly on the screen the surface tension expressed in mN/m according to the Whilhelmy method (Whilhelmy glass plates give quicker and simpler results, but less accurate as those obtained with a du Nouy ring). The tension probe, either the ring or the plate, is lifted from the liquid by manually lowering the measuring vessel on the lift stage.
The electronic micro-balance, which measures the tension force applied by the parly-immerged probe, is the heart of our tensiometer and its accuracy and reproducible operation are optimal. Because the forces acting upon the surfaces and interfaces changes extremely fast, our balance is capable of swift response to the lowest force changes. The maximum load of our balance is 550 mN (60 g).
Standard measuring cups are 50 mm in diameter and the sample temperature can be regulated by a means of our Pt 100 thermoprobe interfaced to a microprocessor-controlled bath/circulator. If sample thermostation is not needed, optional plates or vessels up to max. 120 mm in diameter can be placed on the stage. Standard features include temperature measurement from the water jacket or optionally the temperature is measured directly from the sample by our Pt 100 special needle-like thermoprobe. To provide maximum insulation and to prevent heat loss the water jacket is made of solvent resistant borosilicate glass. Temperature range can be from -10 to +100 �C. A magnetic stirrer can be used. Snap-in, precision water connectors make tube removal from the water jacket easy. Wide opening transparent doors provide easy access to the measuring chamber and protect the sample and probe from air borne particles and draft.
Pulling and lowering of the probe (i.e. ring, plate, fiber etc.) is arranged by moving the liquid sample vertically while the probe is stationary. The measuring cup is placed into the thermostated jacket fixed onto a stage. The vertical movement of the stage can be powered by a high accuracy motor to ensure smoothest possible ride of the stage. The rotational power of the motor is converted into vertical movement via tooth belt which turns the precision screw. The screw moves the stage shaft up or down depending on the direction of the screw rotation. All the stage movements and speeds are software controlled and user programmable. The length of shaft movement is 150 mm and speed is adjusted in 0.01 mm increments within speed range from 0.0000001 to 21 mm/sec). An optional manual control key pad may be used to move the cup without computer control.
By using an automatic jack and our softwares, dynamic measurements allow a wider range of applications:
1. Contact Angle of the Liquid-Solid Interface
2. Effect of Liquid Absorbency and Wicking on Textiles, Powders and other Porous Materials
3. Surface Homogeneity of Solid Sheets and Films
Measurement of dynamic contact angles provides information on the properties of surfaces such as porosity and homogeneity, surface topography and reactivity. The method is based on the Wilhelmy plate principle where the solid sample is held by the electro balance and then dipped or pulled from the substance. Therefore the weight of the sample alternates depending on direction of the movement. The force changes obtained are directly proportional to the contact angle. The contact angle is the angle formed by the tangent to the point of contact at the solid/liquid interfaces. As the solid sample penetrates the surface and dips into the liquid a advancing contact angle is determined and the pull of the sample from the substance provides receding contact angle information. The progress of the experiment is expressed in form of graphical buoyancy slope where the x axis represent the immersion depth and the y-axis the force. The contact angles is calculated from the force versus depth information.
Once the total surface tension of the liquid is known, the polar and dispersive components of the liquid surface tension can be determined by measuring the contact angle of the liquid against a non-polar solid such as teflon or polyethylene. With this information, a calculation can be made to characterize the liquid by polarity and surface tension. This can be especially useful when formulating an ink or coating solution to match the polarity of a film or other solid surface and therefore maximize adhesion at the interface.
Dynamic Contact Angle Analysis is one of the simplest, yet most powerful techniques available for characterizing the top ten angstroms of a solid surface. When a liquid comes in contact with the surface of a solid, the liquid will either spread out to a certain degree onto the surface and thus wet or partially wet the surface, or it will form a cluster or ball of liquid that repels or de-wets the surface.
It is the nature of this interface between a solid and a liquid that is probed by the Dynamic Contact Analyser and provides important insight into the chemistry or wetting thermodynamics of the surface. A good practical example of wettability at work in everyday life is the painted surface of your automobile. When drops of rain fall onto the hood of a newly waxed car, the drops form beads that can easily roll off the hood when it is tilted at an angle. The same water drops on an unprotected car hood will form small puddles of water that streak and do not readily roll off the surface. In the first case, the car with the waxed surface is protected by an organic hydrophobic coating (the wax) that repels water and promotes droplet formation. The unwaxed car has a surface ~ that is more hydrophilic because the unprotected painted metal surface is more wettable than the wax covered surface. Thus, water will tend to spread out rather than form beads on an unwaxed metal surface.
Critical Micelle Concentration (CMC): Surface active molecules, surfactants, concentrates at the surfaces or interfaces of liquids. Surfactant molecules have polar and non-polar ends. At the interface the end of the molecule least compatible with the molecules in the bulk of the liquid orients itself to the next phase. By adding surfactant molecules into the solvent they at some certain concentration form a monolayer at the interface. If concentration is increased beyond this point the surfactant molecules are forced into the bulk of the solvent where they start forming molecule groups called micelles. After the formation of the monolayer the surface tension stabilises. This stabilising point is called CMC, and it is characteristic to a given surfactant. We supply an optional CMC software. The whole measuring process is fully automatic and you can use either plate or ring method. The programming of all dispenser operations, stirring activation, balance adjustments, stage movement and temperature data are entered into program by filling out the pull down experimental set up window.
Adsorption measurements are used to study wetting behaviour of porous solids like medicine tablets, paper and pulp products etc. The sample is hooked to the balance and the liquid is raised until it slightly touches the sample resulting in a significant increase of force. After the contact of the solid and the liquid the weight of the sample increases as the liquid climbs up its surface. The focus of the interest of this specific measurement is in how long it takes until a constant contact angle is formed in between the solid sample and the liquid.
Our optional adsorption software has the following characteristics:
1. measurement of advancing and receding contact angle
2. linear or logarithmic graph presentation
3. weight progression vs. time
4. data acquisition in linear or logarithmic time intervals
Our basic TSD 100 is particularly suitable for beginners and for education. Surface and interfacial tension values are displayed digitally. The selection of measuring methods is simple and calibration is implemented using standard weights.
Applications for the TSD Models.
There are numerous applications, they are used for example to determine and test physical properties of solutions, organic and inorganic liquids, liquid/liquid or liquid/solid dispersions, emulsions, etc. or to develop and test surface active substances such as surfactants and emulsifiers and to identify the such substances in waste water and other waters, for instance:
1. To control cleaning or drinking water. Surfactant traces left from cleaning agents can positively or negatively affect foam formation, e.g. in drinking water, distilled water, beers�, and must be prevented. A surfactant analysis takes lots of time and is quite expensive. The solution is to determine whether traces of surfactant are present in the rinsing water by measuring the surface tension with the TSD 100, by using the ring method, and then comparing it with that of pure water.
2. To distinguish 2 polymer films which are made from the same material and both look and feel the same, but one is less prone to static electricity and can be coated more evenly than the other.
3. To investigate why a carbon fiber/ resin composite made with a plasma treated carbon fiber forms an adhesive bond with the resin which is 40% stronger than an equivalent bond with the same untreated carbon fiber.
4. To discover why two lines of hard contact lens products using the raw material from the same source give not the same comfort to the eye, requires different cleaning, and can be worn for different durations.
5. To understand why old and new ink formulations supplied by the same company show different running behaviour when applied to coated board materials, although they only have a minor difference in one additive to be safer towards the environment.
6. To find why 2 tablets of pain reliever that are made using the same active ingredients show different acting speed and swallow difficulty.
The answer to all these industrial secretes are all traceable to surface tension and wetting properties that are invisible to the naked eye, and in some cases even invisible to sophisticated high vacuum surface analysis instruments like spectrometers.
The tension surface measurement is more and more spreading as a technique for production control and in R&D sector.
Surface wetting phenomena are a subject of a great interest and the applications are very numerous:
Surfactants:
Surfactants have many uses. Depending on the use the surfactant can be called by many names such as Wetting Agents, Emulsifying Agents, Solubilizing Agents, and Detergents. The body is filled with naturally occuring surfactants called phospholipids which are a key component in cell membranes.
When two immiscible liquids are in contact, they tend to maintain as small a surface as possible. It is therefore difficult to mix these two liquids and have them stay mixed. A good example of this is an oil and vinegar salad dressing. The addition of a proper surfactant will lower the interfacial tension between the two liquids and allow them to mix. Creamy salad dressing contains egg yoke or an artificial surfactant to do just that.
Pharmaceuticals:
Surfactants are widely used in pharmacy. Wetting agents are added to suspensions, both oral and parenteral, to hinder caking of particles during storage. Powdered suspensions for reconstitution contain wetting agents to facilitate rapid suspension of the particles upon the addition of a vehicle such as water. Surfactants have even been added to tablets to aid in the penetration of moisture into the tablet to hasten its disintegration.
The hydrophobicity/hydrophilicity of an orally-ingested or transdermally applied drug products is an important factor, especially in the race to develop safe and effective time-released pharmaceuticals.
Paint and coatings:
With the increase use of plastic and new paints with special surfactant mixtures new substrate preparation method must be developed to insure long adhesion;
Textiles:
Every fibers involves surface treatements providing important barriers for protection from the elements;
Cosmetics:
Shampoo and other hair care products are only effective when the surface of the hair is receptive to the product;
Paper, film and ink products:
Surface tension mesurement allows test of wettability and adhesion of every kind of ink;
Control of biomaterials:
For contact lenses, catheters, dental prosthetics and biocompatible implants.
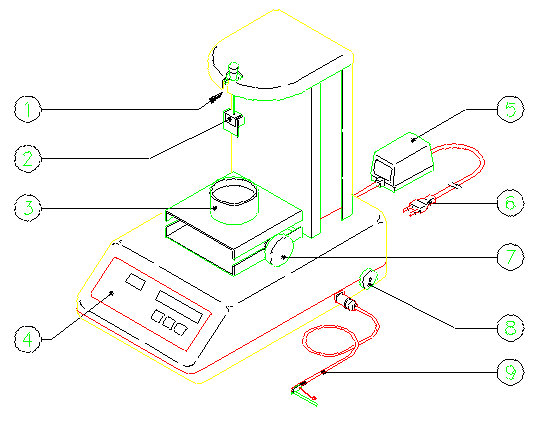
Figure 1
1 - Hangdown
2 - Suspended system for arrangement of glass plate or ring or density plunger or weighing pan
3 - Sample container
4 - Keyboard
5 - Power supply
6 - Power supply plug
7 - Manual adjustable lab jack
8 - Locking device for transport
9 - Thermoprobe
TSD 971 TENSIOMETRY SYSTEM DIGITAL